Objective: To investigate the safety and efficacy of Avapritinib in pediatric acute myeloid leukemia (AML) with KIT mutation after allogeneic hematopoietic stem cell transplantation (allo-HSCT).
Methods: The clinical data of 6 AML patients with KITmutation and RUNX1::RUNX1T1 fusion gene positive in Children's Hospital of Soochow University were retrospectively analyzed, including 2 cases of KIT:N822K, 3 cases of KIT: exon17 and 1 case of KIT: Asp816Tyr. After achieving complete remission in morphology, allo-HSCT was performed. The clinical outcomes of prophylactic therapy (2/6) and preemptive intervention (4/6) with Avapritinib after complete stem cell engraftment were observed, and the adverse events during treatment were recorded.
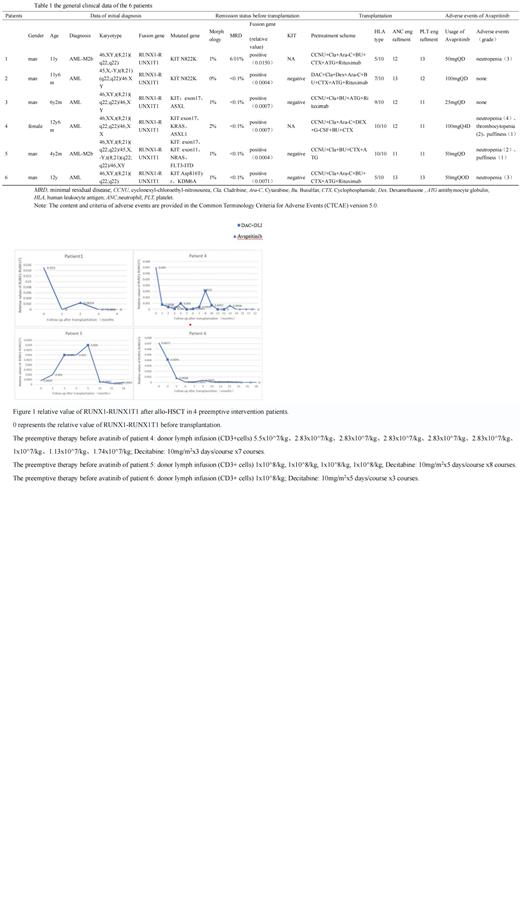
Results: Adverse events occurred in 4 of the 6 patients, among whom neutropenia was the most common (4/6), followed by facial edema (2/6) and thrombocytopenia (1/6). 2 patients who received prophylactic treatment achieved morphological complete remission, and minimal residual disease (MRD), fusion gene and KIT mutations were all negative. 4 patients received preemptive intervention because of RUNX1::RUNX1T1 fusion gene turned positive after allo-HSCT, and 1 patient's RUNX1::RUNX1T1 turned negative after single-agent Avapritinib treatment for 2 months. 2 patients did not achieve continuous RUNX1::RUNX1T1 negative after preemptive therapy with Decitabine (DAC) and donor lymph infusion (DLI), and then was treated with Avapritinib, one's RUNX1::RUNX1T1 fusion achieved continuously negative after 1 month treatment of Avapritinib, the other's achieved continuous negative after 7 months treatment of Avapritinib. One patient was treated with Avapritinib after DAC and DLI as preemtive intervention, and RUNX1::RUNX1T1 fusion did not turn negative, but the relative value of RUNX1::RUNX1T1 fusion was lower than before and lived stable for more than 10 months.
Conclusions: Avapritinib is safe and effective in the prophylactic and preemptive treatment of AML with KIT mutation after allo-HSCT in children, which provides a clinical drug for the prevention of relapse after transplantation.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal